Reaction Thermodynamics
Reaction Thermodynamics helps in evaluation of main thermodynamic parameters of a chemical reaction.
Application calculates the following parameters:
- Standard free Gibbs energy, enthalpy and entropy from formation energy parameters.
- Reaction quotient (Q) from concentrations, pressures and reaction stoichiometry.
- Non standard energy parameters (dG, dH, dS) from standard ones, quotient and temperature.
- Reaction equilibrium constant, Keq.
- Vant Hoff relation to follow change in equilibrium constant with temperature assuming constant reaction enthalpy.
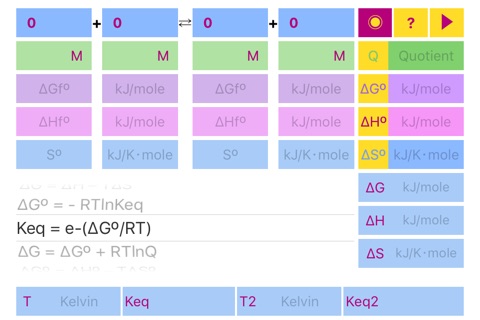
List of Formulas:
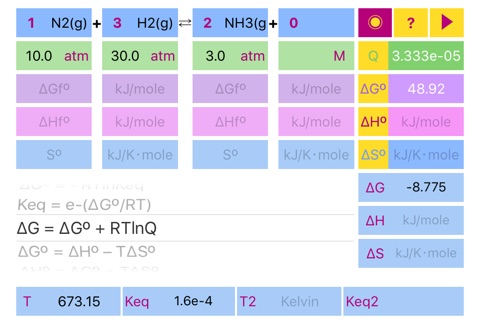
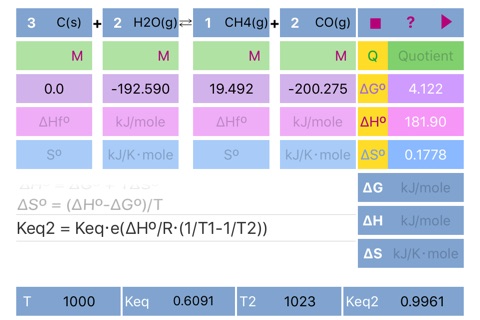
Quotient = Π(C, P)
ΔGº = Σ(ΔGºf)
ΔHº = Σ(ΔHºf)
ΔSº = Σ(Sºf)
ΔG = ΔH – TΔS
ΔGº = - RTlnKeq
Keq = e^(-ΔGº/RT)
ΔG = ΔGº + RTlnQ
ΔGº = ΔHº – TΔSº
ΔHº = ΔGº + TΔSº
ΔSº = (ΔHº - ΔGº)/T
Keq2 = Keq⋅e^(ΔHº/R⋅(1/T1 - 1/T2))"
Scrolling to the required formula and pressing run button initiates calculation according to the formula and set results in the appropriate fields ((dG°, dH°, dS°, Keq, dG, dH, dS, Keq2) and in the fields for standard parameters. Please pay attention to the parameters needed for solution of the formulas.
If formation parameters are unknown, the standard parameters can be filled in directly.
The default units are kJ/mole for dG and dH, kJ/Kmole for dS. Keq units depend on reaction stoichiometry and may be unitless or any mixture of Molar and atm.
Important!!! If compound doesnt participate in equilibrium calculation (like in case of water in water solution or solid in gas phase reaction) please set concentration of the compound to 1 or set its stoichiometric coefficient to 0 (depends on case) to ensure proper Quotient calculations. Ask developer directly in case of a ambiguity.
Reaction stoichiometric parameters and concentration/pressure units can be changed by pressing the corresponding fields.
For further details please refer to www.volard.wordpress.com